
I agree Our site saves small pieces of text information (cookies) on your device in order to deliver better content and for statistical purposes. You can disable the usage of cookies by changing the settings of your browser. By browsing our website without changing the browser settings you grant us permission to store that information on your device.
We have developped a preclinical pipeline and thanks to the viral particles, COVID-19 mouse models, cohort production and BSL3 pipeline analysis we provide to you, you will be bale to assess a novel potential vaccine and treatment compounds for COVID-19. Further functional analysis and advanced readouts like organ histopathological analysis, multiplex assay profiling of cytokines and chemokines in lung and mouse serum etc. can be provided upon request too. A final infection profile report will be provided as a deliverable.
See our Anatomopathology dedicated page to explore all our available tests.
The measurement of various blood metabolites, ions, and enzymes provides a primary screen for function of major metabolic organs such as kidney, liver, gastrointestinal tract, as well as for lipid and glucose homeostasis.
A panel of parameters is proposed, including:
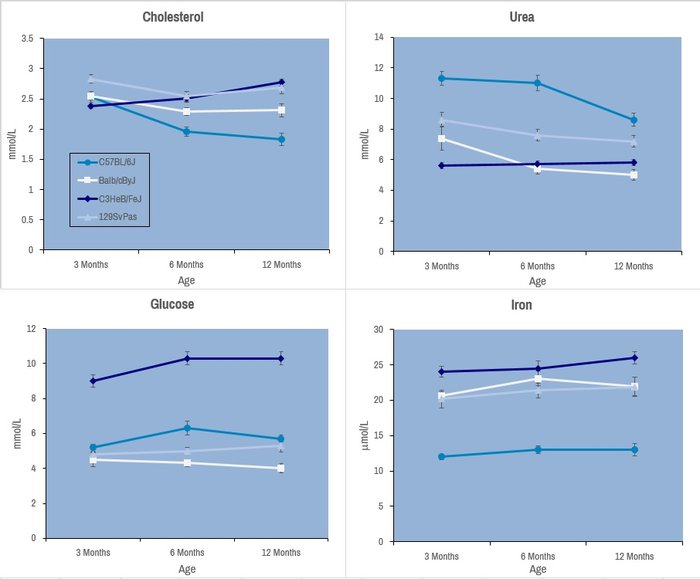
Selected plasma chemistry data from female mice (N=20 per line) of common laboratory strains at different ages (Champy et al. Mammalian Genome 2008). Error bars reflect ± SEM.
These tests are performed with an AU-480 automated laboratory work station (Beckman Coulter France SAS, Villepinte, France).
Wound healing is a complex process requiring cell proliferation, extracellular matrix synthesis, inflammation, and contraction. The biological processes involved can reveal defects in immune response, nutrition, metabolism, or cell division. Wound healing analyses are performed on skin, which is a large and accessible organ, using full thickness excision method.
At least, 15 mutant mice versus 15 littermate control mice are required for relevant results.
Gross evaluation (healing rate and wound aspect evaluation)
At each time point:
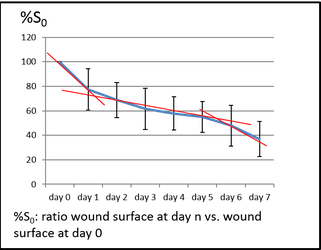 Reference curve for ratio wound surface
Reference curve for ratio wound surface
Histological evaluations
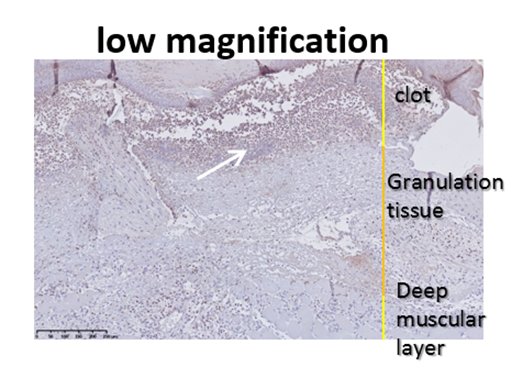 Anti-neutrophil staining (red) after wound healing.
Anti-neutrophil staining (red) after wound healing.
See our Immunoprofiling dedicated page to explore all our available tests.
Controlling infectious diseases requires understanding of the relationships between immune cells and pathogens.
Most current studies to understand infectious processes rely on models requiring
The Biosafety Level 3 Containment Facility (BSL3) has special engineering and containment features that allow investigators to work safely with pathogens classified as risk group 2 or 3. This facility consists of three laboratory suites corresponding to an animal housing room, a laboratory for in vitro experiments and a bio-imaging area.
All instruments are accessible to trained users based on the scientific and regulatory rules established by scientific and biosecurity and safety manager of BSL3. Although the BSL3 platform was only fully operational in May 2015, it already permitted to set up
Real-time imaging studies of immune responses in mice challenged by pathogens such as Salmonella or Brucella infection have led to the development of an immunophenotyping analytical pipeline devoted to infection in synergy with our Immunophenotyping module. The analysis of organ distribution of biomolecules engineered to transport and deliver nucleic acids on living animals using an IVIS Lumina III device is also developed in the frame of a contract with a biotech company. Therefore, we can visualize the spread of infection in vivo, isolate and characterize the pathogens, and analyze phenotypic and functional modification of immune system in the course of infections by advanced live-imaging and flow and mass cytometry.
We provide a broad range of instruments and services for academic and industrial customers. An experienced staff of scientists, technical experts and instrument specialist provides instructions for the design, execution and analysis of standardized multiparametric flow, spectral, mass cytometry and advanced microscopy based studies. All these cutting-edge equipment are technically and operationally enrolled in a regular maintenance program with their commercial providers. Moreover, instrument specialists at PHENOMIN are dedicated to the maintenance and tuning of the instruments on a daily basis. The characterization of the cytometer status and performance using CS&T beads for cytometer set up and tracking system, ensure reproducible performance and instrument sensitivity on a day to day basis. All experiments are standardized using applications setting in order to achieve: (1) high consistency of results over time and (2) facilitate data analysis and results validation. All data are archived and stored on dedicated servers. Depending on the technical complexity and availability, the equipment can be handled by our experts or made available for use to trained customers. PHENOMIN has set up an on line instrument tutorial for training external users. PHENOMIN applies two types of price: one for its academic clients on full cost basis and the other one for its industrial customers taking into consideration intellectual property and the transfer of know-how.
Real-time imaging studies of immune responses in mice challenged by pathogens (Salmonella infection model) and infection-immunophenotyping analysis pipeline in synergy with immunophenotyping CIPHE facility.
Set up vaccine assays and test the ability of adjuvants to enhance vaccine efficacy in a mouse model of infection.
Exploration of airway inflammation and/or airway responsiveness in House Dust Mite-induced lung inflammation.
Advia120
Cytospin 4
Whole-body barometric plethysmograph (EMKA Technologies, Paris, France)
Number of animals - inflammation: 8 mice/gender/genotype
Number of animals - airway responsiveness: 12 mice/gender/genotype
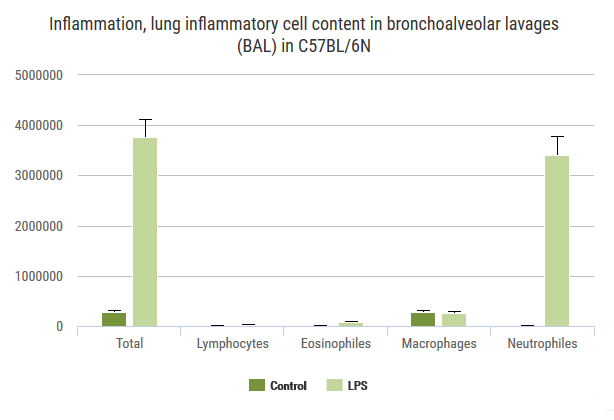
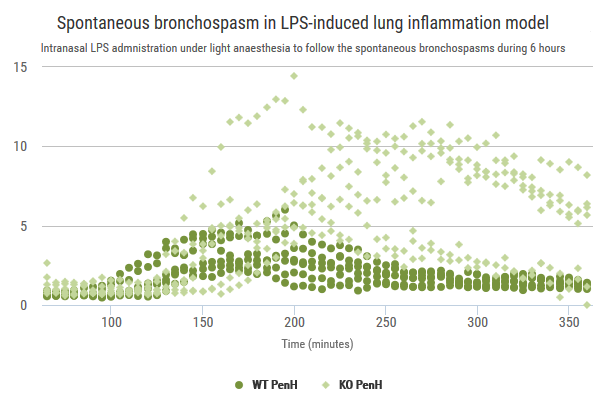
Exploration of airway inflammation and airway responsiveness in LPS-induced inflammation model.
Advia120
Cytospin 4
Whole-body barometric plethysmograph (EMKA Technologies)
Number of animals - inflammation: 8 mice/gender/genotype
Number of animals - airway responsiveness: 12 mice/gender/genotype
We are able to induce eosinophilic inflammation of the lung by presensitizing then exposing the lung to ovalbumin. This can be monitored by whole-body plethysmography, and examined terminally by cytology and cytokine analyses of BALF, immunoglobulin levels in blood and histology.
Identification of specific bacterial loads present in the feces of mice by virtue of quantitative ribosomal 16S DNA sequencing.
For more details, please see our Microbia profiling dedicated section.
See our Gene expression analysis dedicated page to explore our different related tests.
See our In vivo Viral Transduction dedicated page to explore our frequently used vectors and delivery modes including stereotactic admininistration (intraventricular and cerebellar)
With several months notice, we can procure and provide a variety of different restriction diets for nutritional analyses in conjuction with our other analyses.
Bioluminescence imaging is based on the detection of light emitted as a result of an enzymatic reaction involving a couple “enzyme / substrate”, usually “luciferase / luciferin”. When the enzyme is introduced into systems that are not naturally bioluminescent (cells, bacteria, transgenic animals), these systems play the role of reporter gene and allow to address many biological issues such as gene expression, cell proliferation (cancer) or bacterial dissemination (infectious diseases), for example. A dedicated camera collects emitted photons and the signal is processed to obtain images.
Bioluminescence is performed with IVIS Lumina systems and can be carried out under BSL3 conditions.
Some examples of applications :
IVIS Lumina III (Perkin Elmer)
Fluorescence is based on the excitation of a fluorophore then detection of emitted light. The incident light illuminates the fluorophore with one given wavelength that in return emits photons of longer wavelengths. Thus, fluorescence imaging needs a light source to excite the fluorophore within the targeted tissue, combined with a spectral filter to select the excitation wavelength, a second filter that only allows passage of the emitted fluorescence and finally a detector that detects the signal.
Fluorescence is performed with IVIS Lumina systems as planar epi-fluorescence mainly in the near infra-red wavelengths. Imaging can be performed in vivo (superficial foci) or ex vivo (organs).
Some examples of applications:
Dextran-sulphate sodium salt (DSS) provision in drinking water elicits a reversible colonic abrasion and inflammation. This is proposed as a first-line examination where inflammatory stimulation may be dysregulated.
Exploration of airway inflammation and/or airway responsiveness in House Dust Mite-induced lung inflammation.
Advia120
Cytospin 4
Whole-body barometric plethysmograph (EMKA Technologies, Paris, France)
Number of animals - inflammation: 8 mice/gender/genotype
Number of animals - airway responsiveness: 12 mice/gender/genotype
Exploration of airway inflammation and airway responsiveness in LPS-induced inflammation model.


Advia120
Cytospin 4
Whole-body barometric plethysmograph (EMKA Technologies)
Number of animals - inflammation: 8 mice/gender/genotype
Number of animals - airway responsiveness: 12 mice/gender/genotype
We are able to induce eosinophilic inflammation of the lung by presensitizing then exposing the lung to ovalbumin. This can be monitored by whole-body plethysmography, and examined terminally by cytology and cytokine analyses of BALF, immunoglobulin levels in blood and histology.
With several months notice, we can procure and provide a variety of different restriction diets for nutritional analyses in conjuction with our other analyses.
See our In vivo Viral Transduction dedicated page to explore our frequently used vectors and delivery modes including stereotactic admininistration (intraventricular and cerebellar)