
I agree Our site saves small pieces of text information (cookies) on your device in order to deliver better content and for statistical purposes. You can disable the usage of cookies by changing the settings of your browser. By browsing our website without changing the browser settings you grant us permission to store that information on your device.
The Histopathology and Embryology facility provides comprehensive histopathological analyses of mutant or treated mice and rats. Our platform heads are experts in mouse developmental biology able to provide conclusive interpretation of anatomical and cellular changes in your model. Various histological procedures are applied to detect and systematically analyze organ defects and tissue alterations in mice and rats at precise stages of their development as well as in adult and ageing animals. The facility also performs analyses of cell proliferation, apoptosis and immunohistochemical assays using commercially available antibodies.

|
CARDIOVASCULAR SYSTEM |
Heart, Aorta |
|
RESPIRATORY SYSTEM |
Trachea, Lungs |
|
DIGESTIVE ORGANS |
Liver, Pancreas (exocrine), Salivary glands |
|
DIGESTIVE TRACT |
Esophagus, Stomach, Duodenum, Ileum, Colon |
|
CENTRAL NERVOUS SYSTEM |
Brain |
|
SENSORY ORGANS |
Eye and Adnexia, Tongue |
|
ENDOCRINE SYSTEM |
Pituitary, Thyroid and Adrenal glands, Pancreas (endocrine) |
|
IMMUNE and HAEMATOPOIETIC SYSTEMS |
Thymus, Spleen, Lymph nodes, Bone marrow |
|
URINARY SYSTEM |
Urinary bladder, Kidney |
|
MALE REPRODUCTIVE SYSTEM |
Testis, Epididymis, Prostate, Seminal vesicles, Preputial glands |
|
FEMALE REPRODUCTIVE SYSTEM |
Ovaries, Oviducts, Uterus, Vagina |
|
MUSCULOSKELETAL SYSTEM |
Striated muscles, Knee joints |
|
SUPPORTING and CONNECTIVE TISSUES |
White and Brown adipose tissues |
|
INTEGUMENTARY SYSTEM and MAMMARY GLAND |
Skin and appendages, Mammary gland |
|
Group A
3 mutant males
3 mutant females
1 wild type male
1 wild type female
|

|
Age groups
Young ~ 4 months
Old > 12 months
N = 24
|

|
Group B
3 mutant males
3 mutant females
5 wild type males
5 wild type females
|
|

|
|||
|
Systematic macroscopy
Systematic histology
|

|
Report
|

|
Systematic macroscopy
Targeted histology
|

|
|

|
||
|
Paraffin tissue bank
|
Targeted histology
|

|
Transient collection of mummies
|
Cell death by apoptosis can be investigated in ex vivo sections by TUNEL staining (Terminal deoxynucleotidyl transferase-mediated dUTP Nick End Labeling) and/or cleaved caspase 3 immunohistochemistry. TUNEL represents the standard assay for detecting apoptotic cells on histological sections.
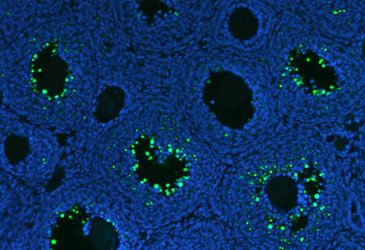 Apoptotic granulosa cells (green dots) in atretic ovarian follicles
Apoptotic granulosa cells (green dots) in atretic ovarian follicles
When comparing apoptosis between different tissues, all samples are fixed and processed under the same conditions. At least, 3 mutant mice versus 3 littermate control mice are required for relevant results.
The lacZ reporter is an effective tool for visualizing gene expression patterns, particularly in development. We offer a full range of services from full-mount embryos to adult sections to examine lacZ expression.
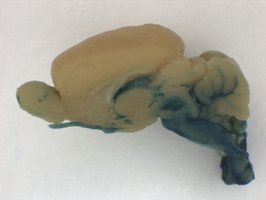 whole mount Lac Z staining of an adult brain
whole mount Lac Z staining of an adult brain
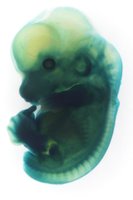 Whole mount Lac Z staining of an E12.5 stage embryo
Whole mount Lac Z staining of an E12.5 stage embryo
Tissue cell proliferation markers provide information on cell cycle progression with BrdU as an indicator of cells having passed through S-phase, phospho-H3 for cells in M-phase, and Ki67 for actively cycling cells.
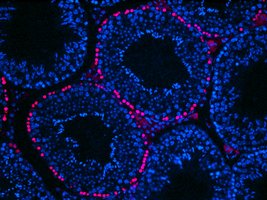 Detection of BrdU incorporation in seminiferous tubules: positive cells are confined to the periphery of the tubules and correspond to spermatogonia and preleptotene spermatocytes close
Detection of BrdU incorporation in seminiferous tubules: positive cells are confined to the periphery of the tubules and correspond to spermatogonia and preleptotene spermatocytes close
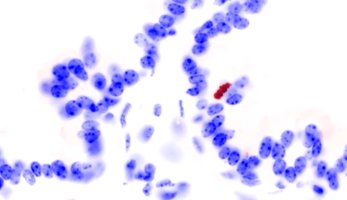 Immunoperoxidase staining for detection of phosphorylated histone H3. Ventral prostate. Negative image obtained by Photoshop® processing of the original, dark-field, pictures. The labeled cell is in metaphase.
Immunoperoxidase staining for detection of phosphorylated histone H3. Ventral prostate. Negative image obtained by Photoshop® processing of the original, dark-field, pictures. The labeled cell is in metaphase.
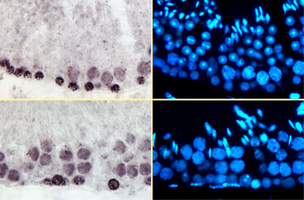 Immunoperoxydase staining for detection of the Ki67 (left panels) and DAPI counterstain (right panels). Stages VII (top two panels) and X (bottom panels) of the seminiferous epithelium cycle.
Immunoperoxydase staining for detection of the Ki67 (left panels) and DAPI counterstain (right panels). Stages VII (top two panels) and X (bottom panels) of the seminiferous epithelium cycle.
To better characterize the primary defects of the mutants, vascular (PECAM), neural (neruofilament), endodermal (HNF3b) markers are proposed. Other marker stainings can be developed on demand.
Wound healing is a complex process requiring cell proliferation, extracellular matrix synthesis, inflammation, and contraction. The biological processes involved can reveal defects in immune response, nutrition, metabolism, or cell division. Wound healing analyses are performed on skin, which is a large and accessible organ, using full thickness excision method.
At least, 15 mutant mice versus 15 littermate control mice are required for relevant results.
Gross evaluation (healing rate and wound aspect evaluation)
At each time point:
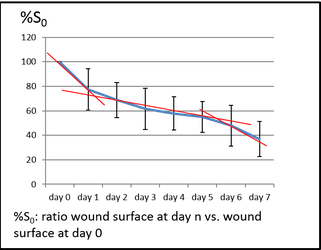 Reference curve for ratio wound surface
Reference curve for ratio wound surface
Histological evaluations
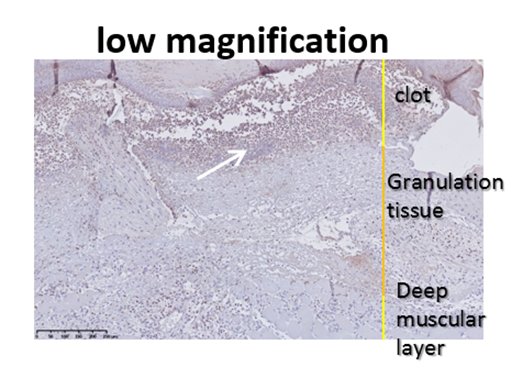 Anti-neutrophil staining (red) after wound healing.
Anti-neutrophil staining (red) after wound healing.
We can provide numerous validated immunohistochemical stains or we can customize protocols to get the best results possible with your antibody of interest.
Example of available immunohistochemical protocols :
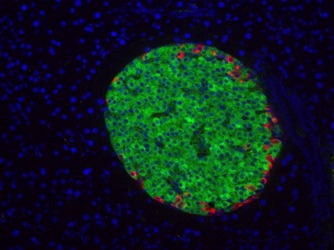 Anti-insulin (green) and anti-glucagon (red) immunostaining on mouse pancreas.
Anti-insulin (green) and anti-glucagon (red) immunostaining on mouse pancreas.
Nucleic acid localization by standard techniques or high-sensitivity branching strategies (RNAscope® by ACDBio) can provide critical gene expression information.
HREM is an effective and powerful tool for assessing structural abnormalities in embryos. Three dimensional reconstruction of embryo slices provides the definition of histological staining with the completeness of 3-D imaging.
HREM imaging is operated with a home made system (developed with the IGBMC Imaging Center)
1-The sample is fixed in Bouin’s solution
2-JB4 Resin embedding :
3-Samples limitations: the size must not exceed 1.5 cm3
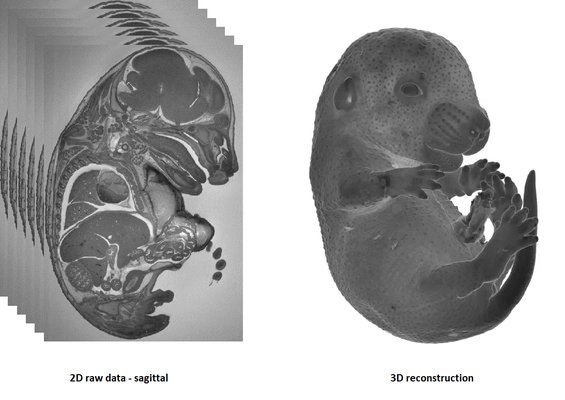
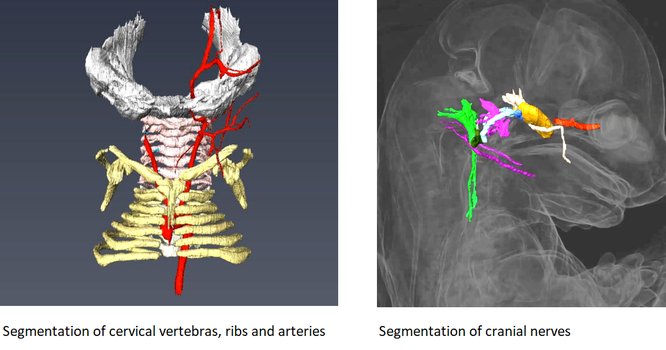
It is recommended to use at least 3 experimental and 3 control samples for analyse.
Rapid three dimensional imaging of density-contrasted tissues (soft tissues) and non-contrasted tissues (skeletal elements) in the embryo using X-ray transmission and computed tomography.
The phenotyping of fetuses at later stages (E18.5) can use micron-scale X-rays Computed Tomography (μCT), which achieves a resolution of 10μm (isotropic voxel size) and allows the morphological analysis of organs in situ without dissection. To achieve sufficient contrast, formalin-fixed fetuses will be stained in Lugol’s iodine as a contrast agent. The impregnation of iodine in tissues will increase X-rays absorption due to iodine’s high atomic number and therefore enhance contrast in the resulting images. Obtained raw data have to be processed to facilitate direct comparison between samples.
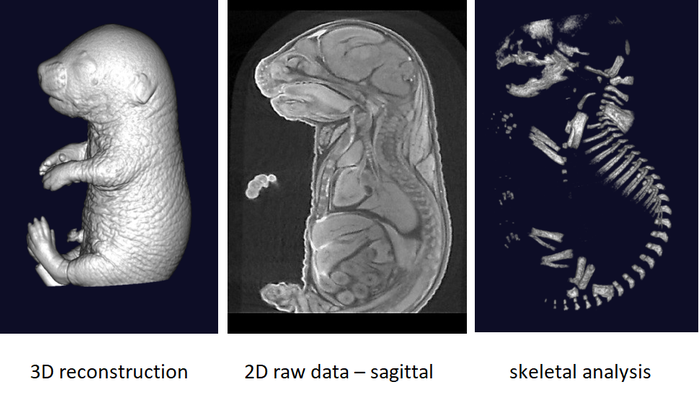
It is recommended to use at least 3 experimental embryos and one control embryo for analyse.
Sampling of embryos for viability at times throughout pregnancy with focus on development of particular organs between E9.5 and E18.5.
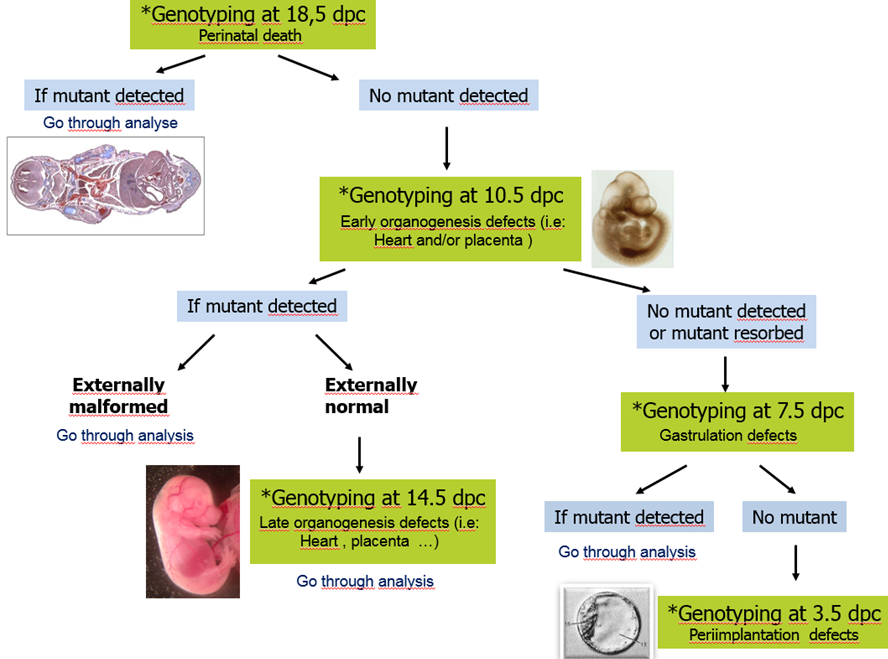 Determination of the window of lethality
Determination of the window of lethality
Nice topographical staining permitting to differentiate bone, blood and mesenchymal structures of the fetuses. Facilitates the analyze.
Embryonic growth restriction can be indicative of placental abnormalities. Macroscopical and routine histological analysis (hematoxylin & eosin) are performed to evaluate placental defects.
Digital imaging of necropsies provides a useful system of post-hoc examination and distribution of macroscopic abnormalities. This is a valuable resource for generation of figures for publication.
The test is performed to examine morphological abnormalities with respect to general physical appearance and body shape on mice: weight, length, and obvious dysmorphologies in the physical appearance,i.e. tail kinks, shape of ears, eyes, head, teethes, limbs, number and shape of digit, irregularities and variation in coat color, hair distribution and development, irregularities in the genitals.
Quantitative measurement of organ sizes for post-hoc determination of potential pathological conditions. Particularly useful in combination with organ storage for post-hoc examinations.
Concurrent staining of bone (alizarin red S) and cartilage (alcian blue) is an excellent strategy for visualizing bone development, particularly in fetuses.
Whole mount mammary glands can be stained with carmine-alum to visualize branching as well as neoplasias.
The gold-standard stain for amyloidosis. It may be viewed without polarization for total amyloid deposits or with polarized light to identify amyloid fibrils.
A one-step stain used to distinguish muscle and collagen fibers in fixed sections.
Haemotoxylin and Eosin is a standard staining technique.
Eosin stains basic structures (including most proteins) in pink and Haemotoxylin stains acidic structures (such as nucleic acids in purple-blue.
Staining of myelin and Nissl substances can be useful for examining neuronal injury or degenerative conditions such as Alzheimers.
This staining is dedicated to distinguish collagen from muscles. Hence, it stains collagens in blue / green and muscles in red.
The diaphorase enzymes present in frozen sections of muscles can be used for enzymatic transfer of hydrogen to a recipient dye for visualization of muscles.
Fat deposits in tissues may be visualized in frozen sections using Oil Red O dye staining.
Orcein is a nature dye that binds non-covalently with elastin and is useful for visualizing elastic fibers in tissues.
Periodic acid oxidation cleaves polysaccharides resulting in aldehydes that can then be covalently bonded to Schiff reagent for visualization. Oxidation reactions must be tightly controlled for quality staining.
Simultaneous discrimination of collagen, elastin, muscle, mucin, and fibrin provides a powerful technique particularly when visualizing heart or vascular abnormalities in fixed tissues.
This staining is often considered as Gold Standard for collagen demonstration. Fibrous proteins are stained in red above a pale orange background. Upon polarized light, collagens are specifically discriminated.
Mast cells will metachromatically stain in violet whereas all other cells will be stained in green/blue (orthochromatic staining).
Tartrate-resistant acid phosphatase stains for osteoclasts that demonstrates some staining of macrophages.
For detection of calcified tissues deposits, metallic silver is visualized at sites of calcium phosphate deposits.