I agree Our site saves small pieces of text information (cookies) on your device in order to deliver better content and for statistical purposes. You can disable the usage of cookies by changing the settings of your browser. By browsing our website without changing the browser settings you grant us permission to store that information on your device.
In vivo imaging modalities detect biological processes in preclinical models with great sensitivity and high resolution. One of the main advantages of In vivo imaging is the possibility to monitor over time and in a non invasive method the evolution of a phenomenon in the same animal. Imaging sets up a unique tool for translational research and exhibits a high potential to accelerate the understanding of diseases as well as to select drug candidates for pharmaceutical development. (
![]() See the French article from the review STAL, Vol47, 2019 (1.4 MB)
)
See the French article from the review STAL, Vol47, 2019 (1.4 MB)
)
We provide researchers with innovative imaging tools and define, together with the teams, the most suitable imaging strategy to address their scientific issue.
|
|
|
MURINE LINES
|
HUMAN LINES
|
|
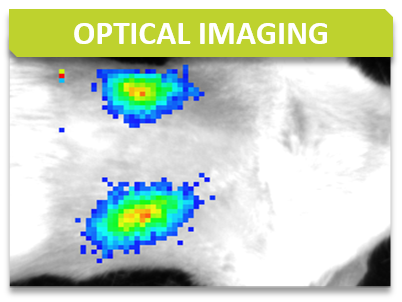
Bioluminescence imaging is based on the detection of light emitted as a result of an enzymatic reaction involving a couple “enzyme / substrate”, usually “luciferase / luciferin”. When the enzyme is introduced into systems that are not naturally bioluminescent (cells, bacteria, transgenic animals), these systems play the role of reporter gene and allow to address many biological issues such as gene expression, cell proliferation (cancer) or bacterial dissemination (infectious diseases), for example. A dedicated camera collects emitted photons and the signal is processed to obtain images.
Bioluminescence is performed with IVIS Lumina systems and can be carried out under BSL3 conditions.
Some examples of applications :
IVIS Lumina III (Perkin Elmer)
Fluorescence is based on the excitation of a fluorophore then detection of emitted light. The incident light illuminates the fluorophore with one given wavelength that in return emits photons of longer wavelengths. Thus, fluorescence imaging needs a light source to excite the fluorophore within the targeted tissue, combined with a spectral filter to select the excitation wavelength, a second filter that only allows passage of the emitted fluorescence and finally a detector that detects the signal.
Fluorescence is performed with IVIS Lumina systems as planar epi-fluorescence mainly in the near infra-red wavelengths. Imaging can be performed in vivo (superficial foci) or ex vivo (organs).
Some examples of applications:
HREM is an effective and powerful tool for assessing structural abnormalities in embryos or organs ex vivo. Essentially ‘3D histology’, a series of successive 2D images of the sample block face is captured during histological sectioning of fixed and embedded tissues in a methacrylate resin. This provides structural information at high resolution with details approaching those conventionally obtained using histological analysis. HREM data can be analyzed either as 2D image stacks or by 3D rendering. HREM volume data can be immediately analyzed using appropriate segmentation and 3d reconstruction softwares.
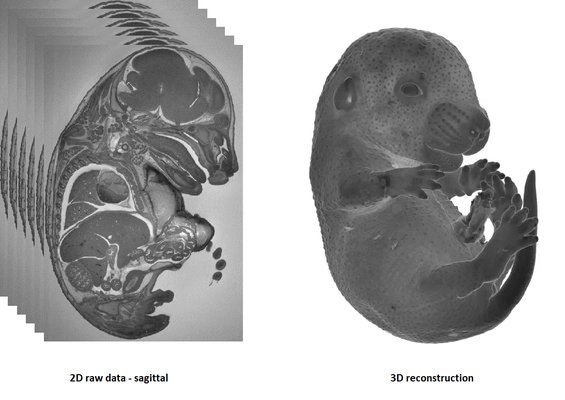
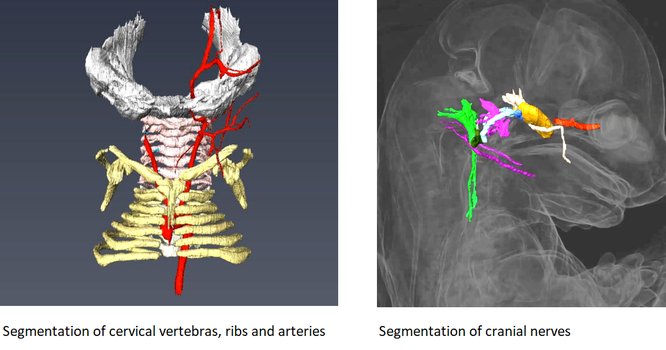
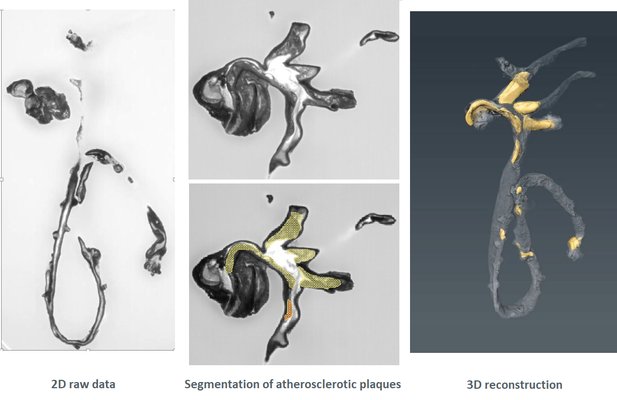
It is recommended to use at least 3 experimental and 3 control samples for analysis.
Photoacoustic imaging is based on the following principle: when producing illuminating laser pulses, lasting several nanoseconds, these light pulses lead to a slight localized heating of the tissue, causing thermoelastic expansion and a subsequent production of ultrasound waves that can be detected on the skin surface.
Some examples of applications:
Photoacoustic imaging is operated with a VevoLAZR device.
Radioisotopic modalities require the use of exogenous radioactive tracers. Depending on radiochemistry and impact on bioactivity, radioactivity can possibly be linked to probe molecules, antibodies, cells, nanoparticles.
These machines can be operated with mice and rats.
Some examples of applications :
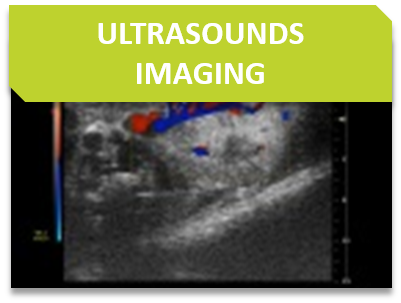
Non-invasive tracking of internal tumor growth using ultrasound or X-ray imaging of tumor density contrasts.
We can provide intratumoral delivery of compounds or genetic vectors without the need for surgery using echographic image-guided injection.
Ultrasound visualizaton of kidneys for non-invasive assessment of tumor growth, mineralization, or cysts.
Orthotopic growth of tumors in soft abdominal organs, i.e. liver, spleen, kidney, can better recapitulate growth conditions of certain tumors. We can provide orthotopic injection without the need for surgery using echographic image-guided injection of cancer cells.
US is an imaging tool that is based on the properties and behavior of high-frequency sound waves as they travel through biological tissues. For such a purpose, a transducer (also called a probe) sends and receives sound waves from the animal.
Some examples of applications:
Ultrasound is performed with a VevoLAZR and some probes are compliant with mice or rats.
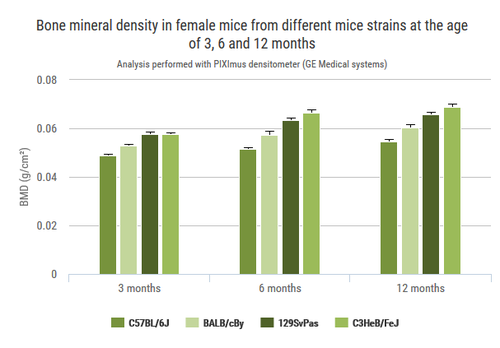
Bone architecture may be analysed by both in vivo and in situ using Micron-scale X-ray Computed Tomography (also called µCT). It is particularly helpful for bone dynamics analysis. This augments our current capacities of skeletal examinations by TRAP histological stain, X-ray, DEXA scanning, quantitative NMR, and adapted clinical chemistry analysis.
µCT (PerkinElmer Quantum FX, Waltham, Massachusetts)
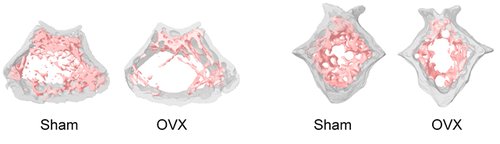
Representative 3D volumes illustrating trabecular microarchitecture in the distal femur (left) and caudal vertebra (right) of Sham-operated and Ovarectomized (OVX) mice, 9 weeks after surgery. Similar volumes are used for morphometric quantification.
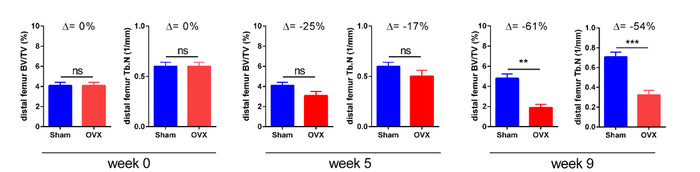
Example of longitudinal changes in trabecular bone volume fraction (BV/TV) and trabecular number (Tb.N) for Sham and OVX groups.
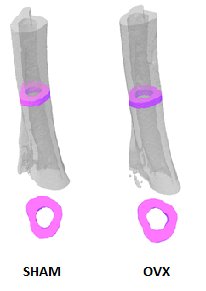
Representative 3D volumes illustrating cortical bone in the tibia of Sham-operated and Ovarectomized OVX mice, 9 weeks after surgery. The illustrations were generated by Alexandru Parlog (PHENOMIN-ICS).
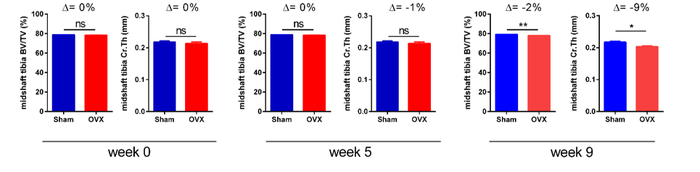
Example of longitudinal changes in cortical bone volume fraction (BV/TV) and cortical thickness (Cr.Th) for Sham-operated and Ovarectomized OVX groups.
Contact us for help in designing your experiments on bone dynamics.
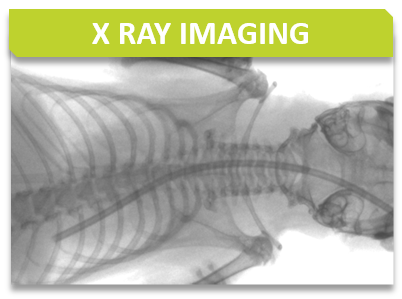
The X-Ray system gives very precise images of the skeletton. In DXA mode it automatically calculates BMD, BMC, and lean and fat mass percentages.

pDEXA Sabre (Norland)
Rapid three dimensional imaging of density-contrasted tissues (soft tissues) and non-contrasted tissues (skeletal elements) in the embryo using X-ray transmission and computed tomography.
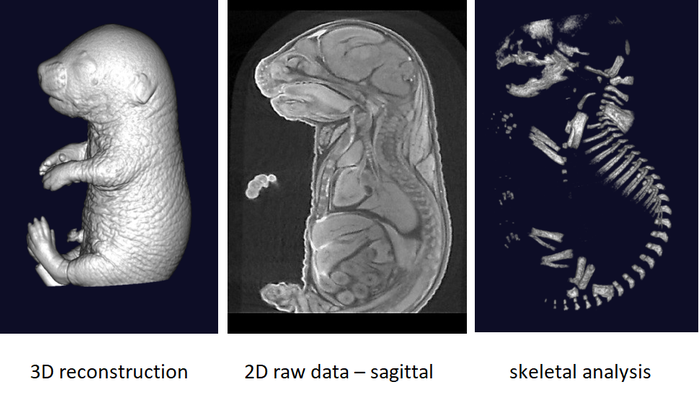
The phenotyping of fetuses at later stages (E18.5) can use micron-scale X-rays Computed Tomography (μCT), which achieves a resolution of 10μm (isotropic voxel size) and allows the morphological analysis of organs in situ without dissection. To achieve sufficient contrast, formalin-fixed fetuses will be stained in Lugol’s iodine as a contrast agent. The impregnation of iodine in tissues will increase X-rays absorption due to iodine’s high atomic number and therefore enhance contrast in the resulting images. Obtained raw data have to be processed to facilitate direct comparison between samples.
It is recommended to use at least 3 experimental embryos and one control embryo for analyse.
CT is a technique that relies on differential levels of X-ray attenuation by tissues within the body to produce images reflecting anatomy.
The assay is developed to provide high resolution images of the whole mouse skeleton. Analysis of the digital X-ray pictures is generally performed with respect to bones from the head (zygomatic bone, maxilla, mandibles), teeth, scapulae, clavicle, ribs (number, shape, fusion), pelvis, vertebrae (numbers, shape and potential fusion of cervical, thoracic, lumbar, pelvic and caudal ones), limb bones (humerus, radius, ulna, femur, tibia), joints, digits and syndactylism.
X-Ray MX-20 Specimen (Faxitron, Tucson, Arizona, USA)

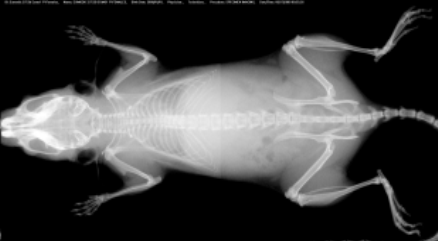
At least 5 mice per group are required for appropriated analysis.