
I agree Our site saves small pieces of text information (cookies) on your device in order to deliver better content and for statistical purposes. You can disable the usage of cookies by changing the settings of your browser. By browsing our website without changing the browser settings you grant us permission to store that information on your device.
PHENOMIN has developed expertise for oncology studies using mouse models for cancer progression and/or initiation. We are available to discuss projects, in close collaboration with users, in order to customize any study to the scientific objectives applying the best adhesion to 3R rules.
We can provide expert advice, define a targeting strategy, and ensure high scientific and technical output in accordance with your needs. Please contact us !
PHENOMIN is regularly performing xenograft models for studying cancer progression. This is performed in syngeneic models, i.e. mouse tumors in isogenic mouse hosts, or human tumors xenografted into immunodeficient mouse models. Syngeneic models are superior for studying the immune response to tumors, whereas the immunodeficient hosts allow a greater array of tumors to be propagated. Syngeneic hosts may also be genetically modified to investigate other aspects of the tumor-stromal interactions. For non-invasive tumor tracking, we have developed a bank of bioluminescent tumor cells of human and murine origin (see listing below).
We currently perform cancer cell-line engraftment by subcutaneous, intravenous, or orthotopic injections. In particular, the development of ultrasound-guided injection offers orthotopic injection of tumor cells without surgery. We have validated cancer-cell injection in liver, kidney, spleen, and pancreas. Ultrasound guidance can also be used to locally inject therapeutic compounds or to realize biopsies.
PHENOMIN is at the cutting edge of mouse genetic models and can generate or access a wide panel of tumor initiation models. As part of the CanPathPro consortium, we are developing a predictive modelization platform for cancer initiation. CanPathPro Flyer
We also have an extensive repository of Cre- ERT2 lines for controlled ablation of genes over time with characterized tissue-specificity.
PHENOMIN is using CRISPR/Cas9 technology as well as homologous recombination techniques for custom model generation. Find out more at genetic engineering and mouse model validation services.
We can apply numerous approaches to answer your scientific questions, including in vivo imaging, pathological investigation, and immunophenotyping.
Tumor growth can be tracked by non-invasive imaging technologies. We have set up a preclinical imaging platform to monitor tumor progression, evaluate new therapeutic strategies by efficacy studies and approach fundamental research in collaboration with biologists and chemists. Our technological platform allows anatomic (X-ray, ultrasounds) as well as functional / molecular investigations (tomoscintigraphy a.k.a. SPECT, positron emission tomography a.k.a. PET, bioluminescence, fluorescence, photoacoustic).
For example, ultrasound imaging allows determination of a tumor’s precise location and volume (2D or 3D scans, for echogenic organs). The X-ray scanner is more appropriate for lungs or bones. We use bioluminescence to follow tumor growth and metastasis with high sensitivity. High sensitivity radioactive and fluorescence imaging techniques, can address a tumor biomarkers’ expression as well as radio- or fluoro-targeted compound screening. Photoacoustic imaging is used to visualize hemoglobin saturation and thus to elucidate the hypoxic dimension of tumors.
PHENOMIN can also provide ex vivo tumor characterization at the cellular or molecular level. Our anatomopathology facility can provide advanced histological staining, standardized immunohistochemical (IHC) characterization for apoptosis (cleaved Caspase 3) and proliferation (Ki67), and custom IHC optimization and testing. We also offer detailed analysis of tumor pathology data for both human and murine tumors.
At the nucleic-acid level, our ddPCR technology enables the detection of DNA or mRNA from very small amounts of tissue. This highly sensitive technique may be applied to rare genetic lesions and / or contrasting expression studies on small tumors or metastases.
In light of the complex tumor microenvironment, complementary experimental approaches have been used to assess the different cell types, their relative proportions, as well as their specificity.
The immunophenotyping is realized by using a combination of antibodies’ panels in flow mass and spectral cytometry. These panels (16 to 36 antibodies) allows the identification of lymphoid and myeloid populations which are present in a given organ or tissue. Specific panels for T, B, DC and NK cells are also validated in association with immune-modulator receptors, cytokines, chemokines and transcription factors.
Regarding pharmacokinetics and pharmacodynamics, we provide PK/PD analysis on plasma samples from peripheral blood collection at different times (various routes of blood collection can be used). The impact of a therapeutic drug is assessed by analyzing cellular composition from several organss from a same animal. Tumor, blood, spleen, lymphatic ganglions are collected to perform high content immunophenotyping; the extensive characterization of tumor and tumor environment infiltrate requires a controlled cellular extraction which preserves surface markers.

See our Immunoprofiling dedicated page to explore all our available tests.
Bioluminescence imaging is based on the detection of light emitted as a result of an enzymatic reaction involving a couple “enzyme / substrate”, usually “luciferase / luciferin”. When the enzyme is introduced into systems that are not naturally bioluminescent (cells, bacteria, transgenic animals), these systems play the role of reporter gene and allow to address many biological issues such as gene expression, cell proliferation (cancer) or bacterial dissemination (infectious diseases), for example. A dedicated camera collects emitted photons and the signal is processed to obtain images.
Bioluminescence is performed with IVIS Lumina systems and can be carried out under BSL3 conditions.
Some examples of applications :
IVIS Lumina III (Perkin Elmer)
Fluorescence is based on the excitation of a fluorophore then detection of emitted light. The incident light illuminates the fluorophore with one given wavelength that in return emits photons of longer wavelengths. Thus, fluorescence imaging needs a light source to excite the fluorophore within the targeted tissue, combined with a spectral filter to select the excitation wavelength, a second filter that only allows passage of the emitted fluorescence and finally a detector that detects the signal.
Fluorescence is performed with IVIS Lumina systems as planar epi-fluorescence mainly in the near infra-red wavelengths. Imaging can be performed in vivo (superficial foci) or ex vivo (organs).
Some examples of applications:
Photoacoustic imaging is based on the following principle: when producing illuminating laser pulses, lasting several nanoseconds, these light pulses lead to a slight localized heating of the tissue, causing thermoelastic expansion and a subsequent production of ultrasound waves that can be detected on the skin surface.
Some examples of applications:
Photoacoustic imaging is operated with a VevoLAZR device.
Radioisotopic modalities require the use of exogenous radioactive tracers. Depending on radiochemistry and impact on bioactivity, radioactivity can possibly be linked to probe molecules, antibodies, cells, nanoparticles.
These machines can be operated with mice and rats.
Some examples of applications :
Non-invasive tracking of internal tumor growth using ultrasound or X-ray imaging of tumor density contrasts.
We can provide intratumoral delivery of compounds or genetic vectors without the need for surgery using echographic image-guided injection.
Orthotopic growth of tumors in soft abdominal organs, i.e. liver, spleen, kidney, can better recapitulate growth conditions of certain tumors. We can provide orthotopic injection without the need for surgery using echographic image-guided injection of cancer cells.
US is an imaging tool that is based on the properties and behavior of high-frequency sound waves as they travel through biological tissues. For such a purpose, a transducer (also called a probe) sends and receives sound waves from the animal.
Some examples of applications:
Ultrasound is performed with a VevoLAZR and some probes are compliant with mice or rats.
CT is a technique that relies on differential levels of X-ray attenuation by tissues within the body to produce images reflecting anatomy.
The assay is developed to provide high resolution images of the whole mouse skeleton. Analysis of the digital X-ray pictures is generally performed with respect to bones from the head (zygomatic bone, maxilla, mandibles), teeth, scapulae, clavicle, ribs (number, shape, fusion), pelvis, vertebrae (numbers, shape and potential fusion of cervical, thoracic, lumbar, pelvic and caudal ones), limb bones (humerus, radius, ulna, femur, tibia), joints, digits and syndactylism.
X-Ray MX-20 Specimen (Faxitron, Tucson, Arizona, USA)

At least 5 mice per group are required for appropriated analysis.
Identification of specific bacterial loads present in the feces of mice by virtue of quantitative ribosomal 16S DNA sequencing.
For more details, please see our Microbia profiling dedicated section.
See our Anatomopathology dedicated page to explore all our available tests.
Cell death by apoptosis can be investigated in ex vivo sections by TUNEL staining (Terminal deoxynucleotidyl transferase-mediated dUTP Nick End Labeling) and/or cleaved caspase 3 immunohistochemistry. TUNEL represents the standard assay for detecting apoptotic cells on histological sections.
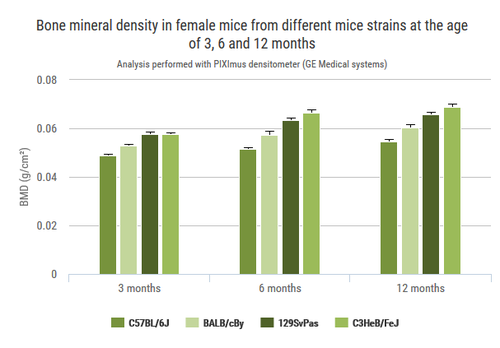
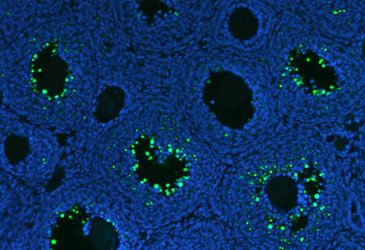 Apoptotic granulosa cells (green dots) in atretic ovarian follicles
Apoptotic granulosa cells (green dots) in atretic ovarian follicles
When comparing apoptosis between different tissues, all samples are fixed and processed under the same conditions. At least, 3 mutant mice versus 3 littermate control mice are required for relevant results.
The measurement of various blood metabolites, ions, and enzymes provides a primary screen for function of major metabolic organs such as kidney, liver, gastrointestinal tract, as well as for lipid and glucose homeostasis.
A panel of parameters is proposed, including:
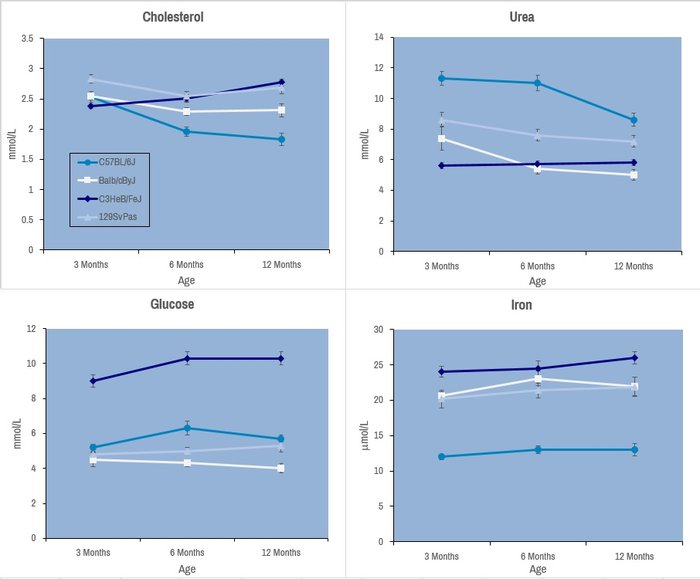
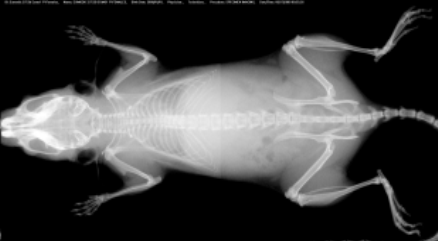
Selected plasma chemistry data from female mice (N=20 per line) of common laboratory strains at different ages (Champy et al. Mammalian Genome 2008). Error bars reflect ± SEM.
These tests are performed with an AU-480 automated laboratory work station (Beckman Coulter France SAS, Villepinte, France).
Tissue cell proliferation markers provide information on cell cycle progression with BrdU as an indicator of cells having passed through S-phase, phospho-H3 for cells in M-phase, and Ki67 for actively cycling cells.
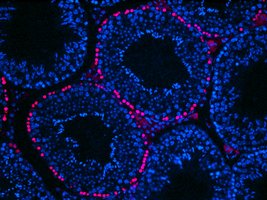 Detection of BrdU incorporation in seminiferous tubules: positive cells are confined to the periphery of the tubules and correspond to spermatogonia and preleptotene spermatocytes close
Detection of BrdU incorporation in seminiferous tubules: positive cells are confined to the periphery of the tubules and correspond to spermatogonia and preleptotene spermatocytes close
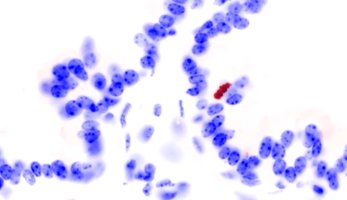 Immunoperoxidase staining for detection of phosphorylated histone H3. Ventral prostate. Negative image obtained by Photoshop® processing of the original, dark-field, pictures. The labeled cell is in metaphase.
Immunoperoxidase staining for detection of phosphorylated histone H3. Ventral prostate. Negative image obtained by Photoshop® processing of the original, dark-field, pictures. The labeled cell is in metaphase.
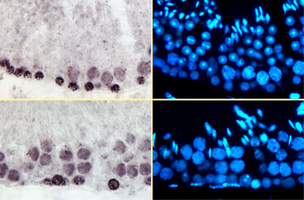 Immunoperoxydase staining for detection of the Ki67 (left panels) and DAPI counterstain (right panels). Stages VII (top two panels) and X (bottom panels) of the seminiferous epithelium cycle.
Immunoperoxydase staining for detection of the Ki67 (left panels) and DAPI counterstain (right panels). Stages VII (top two panels) and X (bottom panels) of the seminiferous epithelium cycle.
See our Gene expression analysis dedicated page to explore our different related tests.
See our In vivo Viral Transduction dedicated page to explore our frequently used vectors and delivery modes including stereotactic admininistration (intraventricular and cerebellar)
Controlling infectious diseases requires understanding of the relationships between immune cells and pathogens.
Most current studies to understand infectious processes rely on models requiring
The Biosafety Level 3 Containment Facility (BSL3) has special engineering and containment features that allow investigators to work safely with pathogens classified as risk group 2 or 3. This facility consists of three laboratory suites corresponding to an animal housing room, a laboratory for in vitro experiments and a bio-imaging area.
All instruments are accessible to trained users based on the scientific and regulatory rules established by scientific and biosecurity and safety manager of BSL3. Although the BSL3 platform was only fully operational in May 2015, it already permitted to set up
Real-time imaging studies of immune responses in mice challenged by pathogens such as Salmonella or Brucella infection have led to the development of an immunophenotyping analytical pipeline devoted to infection in synergy with our Immunophenotyping module. The analysis of organ distribution of biomolecules engineered to transport and deliver nucleic acids on living animals using an IVIS Lumina III device is also developed in the frame of a contract with a biotech company. Therefore, we can visualize the spread of infection in vivo, isolate and characterize the pathogens, and analyze phenotypic and functional modification of immune system in the course of infections by advanced live-imaging and flow and mass cytometry.
We provide a broad range of instruments and services for academic and industrial customers. An experienced staff of scientists, technical experts and instrument specialist provides instructions for the design, execution and analysis of standardized multiparametric flow, spectral, mass cytometry and advanced microscopy based studies. All these cutting-edge equipment are technically and operationally enrolled in a regular maintenance program with their commercial providers. Moreover, instrument specialists at PHENOMIN are dedicated to the maintenance and tuning of the instruments on a daily basis. The characterization of the cytometer status and performance using CS&T beads for cytometer set up and tracking system, ensure reproducible performance and instrument sensitivity on a day to day basis. All experiments are standardized using applications setting in order to achieve: (1) high consistency of results over time and (2) facilitate data analysis and results validation. All data are archived and stored on dedicated servers. Depending on the technical complexity and availability, the equipment can be handled by our experts or made available for use to trained customers. PHENOMIN has set up an on line instrument tutorial for training external users. PHENOMIN applies two types of price: one for its academic clients on full cost basis and the other one for its industrial customers taking into consideration intellectual property and the transfer of know-how.
Real-time imaging studies of immune responses in mice challenged by pathogens (Salmonella infection model) and infection-immunophenotyping analysis pipeline in synergy with immunophenotyping CIPHE facility.
Set up vaccine assays and test the ability of adjuvants to enhance vaccine efficacy in a mouse model of infection.
Determination of systolic blood pressure and heart rate in conscious mice by tail-cuff method.
Two apparatus of BP-2000 seriesII 6 channels (Visitech Systems, Apex, North Carolina, USA)
Number of animals: 9 mice/gender/genotype . Maximum age : 8 weeks
See our In vivo Viral Transduction dedicated page to explore our frequently used vectors and delivery modes including stereotactic admininistration (intraventricular and cerebellar)